
If it is greater than zero, the reaction is product-favored. Now that DS syst and DS surr are known, DS univ can be determined. DH syst can be calculated in the way described in the Thermochemical Equations module. So, q surr and DH syst must always have opposite signs, which is why DH syst is given a negative sign. Why does DH syst have a negative sign? Any heat lost by the system is gained by the surroundings, and conversely, any heat gained by the system is lost by the surroundings.

Thus, equation (1) can be used to calculate DS surr: Since the surroundings are so much bigger than the system, its temperature is certain to stay constant. But, the only way the system affects the surroundings is by a transfer of heat. It may seem unlikely that the entropy change of the surroundings can be calculated just from what is known about the system. It can be calculated using absolute entropies as has been described on the previous page. Is product- or reactant-favored? The entropy change of the universe can beīroken up into two parts, the entropy change of the system and the entropyĭS syst, the entropy change of the system, represents the change in order of the molecules of the system, similar to what was discussed in Entropy 2.
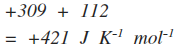
How can the Second Law of Thermodynamics be used to predict whether a reaction The formal statement of this fact is the Second Law of Thermodynamics: in any product-favored process the entropy of the universe increases. The situations described in the second and third pages of this tutorial illustrate the fact that product-favored reactions tend to increase disorder simply because they are much more likely to occur.


 0 kommentar(er)
0 kommentar(er)
